Electrical signaling in fungi: past and present challenges
Summary of Rearch Paper: https://pmc.ncbi.nlm.nih.gov/articles/PMC11995700/ (Download PDF)
By Autors: Matteo Buffi 1, Julia M Kelliher 2,3, Aaron J Robinson 4, Diego Gonzalez 5, Guillaume Cailleau 6, Justine A Macalindong 7, Eleonora Frau 8, Silvia Schintke 9, Patrick S G Chain 10, Claire E Stanley 11, Markus Künzler 12, Saskia Bindschedler 13, Pilar Junier 14,✉
Abstract
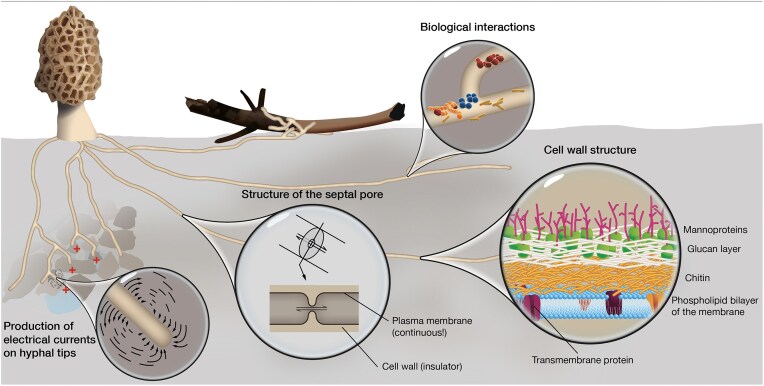
Electrical signaling is a fundamental mechanism for integrating environmental stimuli and coordinating responses in living organisms. While extensively studied in animals and plants, the role of electrical signaling in fungi remains a largely underexplored field. Early studies suggested that filamentous fungi generate action potential-like signals and electrical currents at hyphal tips, yet their function in intracellular communication remained unclear. Renewed interest in fungal electrical activity has fueled developments such as the hypothesis that mycorrhizal networks facilitate electrical communication between plants and the emerging field of fungal-based electronic materials. Given their continuous plasma membrane, specialized septal pores, and insulating cell wall structures, filamentous fungi possess architectural features that could support electrical signaling over long distances. However, studying electrical phenomena in fungal networks presents unique challenges due to the microscopic dimensions of hyphae, the structural complexity of highly modular mycelial networks, and the limitations of traditional electrophysiological methods. This review synthesizes current evidence for electrical signaling in filamentous fungi, evaluates methodological approaches, and highlights experimental challenges. By addressing these challenges and identifying best practices, we aim to advance research in this field and provide a foundation for future studies exploring the role of electrical signaling in fungal biology.
Key Practical Takeaways for Building Fungal Bioelectrical Interfaces
1. Signal Nature and Magnitude
- Low amplitude: Fungal electrical potentials are typically in the nV–µV range; currents are µA cm⁻² — far below animal neurons.
- Low propagation speed: Likely much slower than nerves; closer to plants (cm/sec) than animals (m/sec).
- Signal types: Action potential-like spikes, variation potentials, systemic potentials — possibly linked to environmental stress, nutrient exchange, or predation.
Implication: Your hardware must have very low-noise amplification and good shielding to detect tiny voltages without drowning in environmental noise.
2. Electrode Strategy
-
Placement matters: Not all hyphae are equally active. Signals often occur in:
- Hyphal tips (growth zones)
- Interaction zones (e.g., with plant roots or predators)
- Specialized structures (cords, trunk hyphae)
-
Continuity advantage: Plasma membrane is continuous even if septal pores are closed, so electrical conduction can persist during stress.
-
Insulating cell wall: Hydrophobins and melanins may reduce leakage — a natural benefit for conduction.
Implication:
- Use non-polarizable electrodes (Ag/AgCl or conductive gels) placed in targeted zones.
- Test different locations; expect spatial variation in activity.
- Consider arrays for mapping multiple sites.
3. Noise and Artifacts
- Mycelium is modular and highly dynamic; growth or reorganization can change signal pathways mid-experiment.
- Single-point readings can mislead — local events ≠ whole-network activity.
- Environmental fluctuations (humidity, temp, light) can mimic or mask signals.
Implication:
- Multiple simultaneous channels → avoid over-interpreting one electrode’s trace.
- Log environmental variables in parallel.
- Use Faraday shielding if possible.
4. Measurement Methodology
- Voltage recordings: Capture membrane potential shifts (nV–µV range).
- Current recordings: Detect ionic/electronic flows (µA scale).
- Adapted amplifiers: EEG/ECG-style instrumentation amps with high input impedance.
- Sampling rate: 1–10 Hz is often sufficient; faster for AP-like spikes (~1–100 Hz possible).
Implication:
- An Arduino/ESP32 + ADS1115 (16-bit) or ADS1262 (32-bit) can be an inexpensive starting point.
- For serious work, use a low-noise biopotential amplifier (e.g., TI ADS1299, Intan RHD series).
5. Biological Control & Repeatability
- Mycelium responds to stimuli: nutrient pulses, mechanical touch, light, temperature shifts, predators.
- Controlled stimuli help identify real signals vs baseline noise.
- Growth stage matters — young tips vs mature cords show different patterns.
Implication:
- Design stimulus-response experiments: apply controlled perturbations and watch for consistent electrical changes.
- Keep culture conditions stable and reproducible.
6. Data Interpretation
- Expect localized, event-driven bursts rather than constant “brain-like” chatter.
- Signals may be long-distance but not simultaneous across the network.
- Correlate electrical events with observed physical/biological changes.
Implication:
- Pair electrodes with time-synced imaging (macro or microscope) to see what the fungus was doing.
- Store long continuous recordings (hours–days) to capture rare events.
7. Common Pitfalls
- Misinterpreting drift/temperature effects as bioelectric signals.
- Using unsuitable electrodes (too large, wrong material, high polarization noise).
- Not accounting for mycelium’s 3D geometry and modularity.
Minimum Viable Setup for a DIY Fungal Interface
- Culture: Easy species (e.g., Pleurotus ostreatus) on agar or grain.
- Electrodes: Ag/AgCl, 0.5–1 mm tip; placed into agar just touching hyphae.
- Amplifier: ADS1299-based EEG board or similar.
- Shielding: Basic Faraday cage or grounded metal box.
- Stimuli: Light pulse, nutrient droplet, gentle touch.
- Logging: 1–100 Hz sampling, synchronized with environmental data.
- Analysis: Filter (0.01–10 Hz for slow potentials; higher for AP-like spikes).